Abstract
The developmental switch from fetal-to-adult hemoglobin production is regulated by several transcription factors, including BCL11A, which in turn is expressed predominantly at the adult stage and directly silences the fetal-type globin genes (HBG1/2). Previously, we identified HIC2 as a direct transcriptional repressor of BCL11A in fetal erythroid cells (Huang et al, Nature Genetics, 2022). HIC2 is expressed in a developmental pattern opposite to that of BCL11A with high levels in fetal erythroblasts but extinguished in adult cells. However, the mechanism by which HIC2 expression is controlled during development is unknown.
Potential mechanisms of HIC2 regulation include transcriptional and post-transcriptional control. The HIC2 mRNA contains a long 3'UTR (~4.7kb). As the 3'UTR region may be bound by dedicated micro-RNAs (miRNAs) to influence HIC2 mRNA stability or translation, we investigated the role of miRNAs in HIC2 regulation. Scanning for potential miRNAs target sites within the HIC2 3'UTR revealed a total of 303 candidate miRNAs. Mining published small RNA sequencing data sets from fetal and adult erythroblasts narrowed down 7 miRNAs with relevant developmental expression patterns. All of the 7 adult stage-enriched miRNAs belong to the let-7 family, which are also the top predicted candidates in silico. Let-7 miRNA biogenesis is blocked by the fetal expressed RNA-binding protein LIN28B, and both have been implicated in hemoglobin switching, although their mechanisms of action remain incompletely understood.
To test if HIC2 is regulated by let-7 miRNAs, we forced the expression of let-7a in the fetal-type erythroblast cell line HUDEP1, in which HIC2 levels are relatively high. Both HIC2 mRNA and protein were downregulated upon let-7a overexpression, and, consistent with our previous studies, BCL11A levels were significantly increased. The HIC2 3'UTR has four predicted let-7 seed sequences. Reporter assays in which the HIC2 3'UTR was fused to luciferase demonstrated that while mutation of any single let-7 binding motif had no effect, mutating all four sites strongly increased luciferase signal. This suggests that let-7 acts directly on the HIC2 3'UTR to downregulate HIC2 production.
We inhibited let-7a and let-7f (the most highly expressed let-7 variants in adult erythroblasts) in adult-type HUDEP2 and primary adult erythroid cells via expression of a let-7a/f decoy. This triggered a strong increase in HIC2 mRNA and protein levels, lowered BCL11A expression and increased HBG1/2 transcription. Let-7a/f inhibition significantly decreased the chromatin accessibilities at BCL11A erythroid enhancers, likely a direct consequence of the repressive function of HIC2. Indeed, co-depletion of HIC2 and let-7a/f restored BCL11A and HBG1/2 levels, suggesting that let-7 miRNAs regulate BCL11A and HBG1/2 predominantly via inhibition of HIC2.
Finally, we depleted pre-miRNA processing enzyme DICER1 to globally block miRNA biogenesis in HUDEP2 cells and examined the effects on HBG1/2 regulation. Similar to let-7a/f inhibition, we observed a strong induction of HIC2 and HBG1/2 levels, and a reduction of BCL11A. Co-depletion of HIC2 and DICER1 partially rescued BCL11A and re-silenced HBG1/2 levels, confirming the miRNA mediated post-transcriptional regulatory mechanism of HIC2 expression.
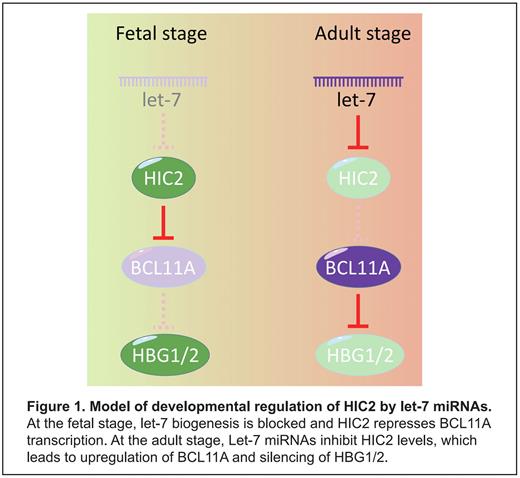
In sum, our study shows that HIC2 is silenced in adult erythroid cells at least in part post-transcriptionally via let-7, and highlights a miRNA mechanism as a means to control the levels of BCL11A and fetal globin genes (Figure 1).
Disclosures
Peslak:Fulcrum Therapeutics: Consultancy. Blobel:Fulcrum Therapeutics: Research Funding; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal